General Mechanism
Luche, J. L. J. Am. Chem. Soc 1978, 100, 2226–2227.
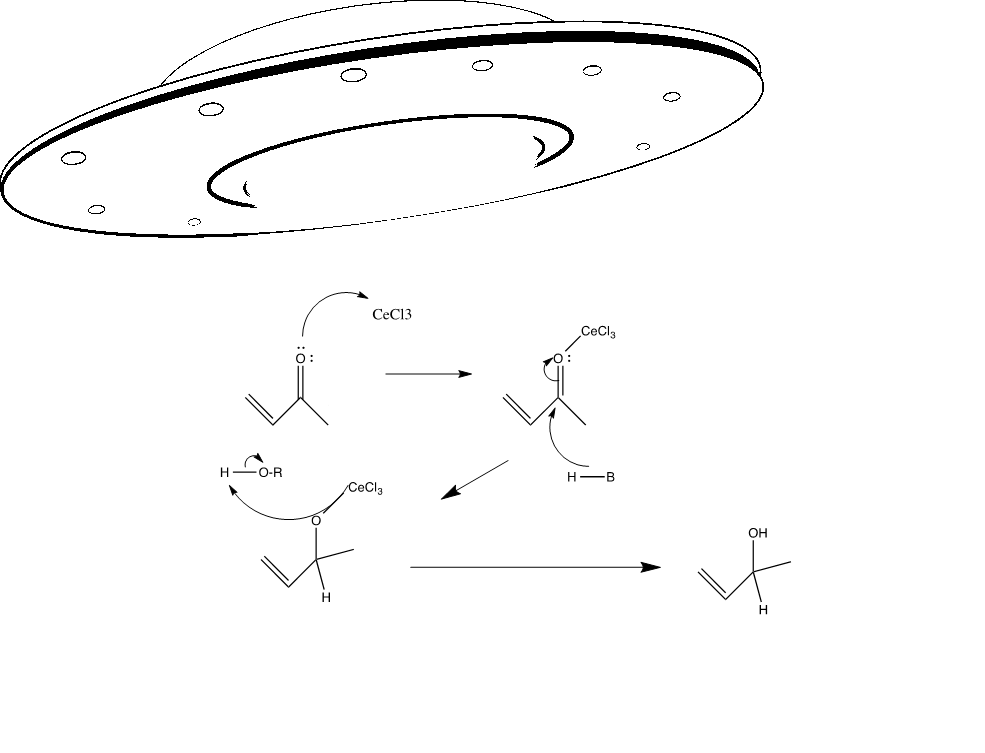
This particular reaction is used to synthesize compound 16 from compound 15.
The Luche reduction is an organic reaction used to convert an α, β-unsaturated ketone to an allylic alcohol using cerium trichloride, sodium borohydride, and an alcohol solvent. The role of the cerium is to coordinate to the alcohol, making its proton more acidic, which allows for it to be abstracted by the carbonyl oxygen of the ketone starting material. The NaBH4 starting regent also reacts with the cerium activated alcohol to form a series of alkoxy borohydrides and these reagents result in the 1,2 hydride attack on the protonated carbonyl to give the final allylic alcohol product. This particular mechanism prepares the molecule 15 to synthesize into 16, so it allows continuing on to the Sn2 reaction to form molecule 17.
|
